Executive Summary
MIGAL’s Coronavirus Vaccine Project
Overview
MIGAL, an internationally-recognized multi-disciplinary applied research institute located in Israel’s Northern Galilee, is developing an oral Coronavirus vaccine that will be ready for in-vivo testing in 90 days. Migal was established in 1979, and today is addressing the world’s most pressing challenges by research in agriculture, food, feed, health and environment.
Based on inter-disciplinary research of groups in the fields of immunology, microbiology, bioinformatics virology enzymology and computational chemistry, Migal is uniquely qualified to carry out this work based on its successful development of a vaccine against Infectious Bronchitis Virus (IBV), an avian (poultry) coronavirus with high similarity to today’s human COVID-19 that uses the same infection mechanism. Given the similarity, and following required genetic adjustments, the same vaccination concepts should apply.
MIGAL’s interdisciplinary vaccine development team has been collaborating for several years on other vaccine development projects, and is highly qualified to carry out this project. Now it seeks partners to help it fund, complete and commercialize its development process.
Background: Coronavirus disease (COVID-19) Pandemic
Coronaviruses are a large family of viruses which may cause illness in animals or humans. In humans, several coronaviruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered coronavirus causes coronavirus disease COVID-19.
COVID-19 is the infectious disease caused by the most recently discovered coronavirus. This new virus and disease were unknown before the outbreak began in Wuhan, China, in December 2019. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
The number of patients worldwide continues to increase: there are large and growing the disease has been reported over countries. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
The highly contagious nature of the disease, coupled with uncertainty regarding its severity and the lack of effective treatment, has led to severe disruptions in the economy and day-to-day functioning of the entire developed world.
The incubation period of the disease is generally 2-14 days, but in some exceptions, it has appeared after 27 days.
Current treatment:
There is currently no certainty as to what drugs could be effective in treating human coronavirus, and no vaccines. A number of drugs currently used in the treatment of patients with other viral diseases, such as AIDS and Malaria (and ebola), are now being used with COVID 19 patients including Remdesivir and Chloroquine, and it is expected that drugs that have been effective for viral diseases (and primarily RNA viruses) are likely to help with recovery.
Vaccine development: Besides Migal, a number of research groups throughout the world are now working intensively to create a vaccine against this virus. Israel’s Biology Institute has engaged a large group of scientists experienced in the development, testing and manufacture of vaccines to work on the project. Moderna has announced the development of a vaccine prototype based on the virus’s genetic material within 4 days of publishing its findings, and will soon begin human trials. GSK is developing a partnership with a Chinese company, and Johnson & Johnson has announced the development of a vaccine and treatments against the disease.
The significant advantage of Migal’s efforts is that its successful 4-year project to develop a vaccine against avian coronavirus has given it a 4-year “head start” which it believes will translate into a rapid development process for an effective human vaccine.
MIGAL’s Corona- New subunit vaccine Development Program
MIGAL initiated its Corona-vaccine development program four years ago with $4 million funding by the Israeli Ministry of Agriculture. As part of the project, MIGAL researchers developed a new sub-unit vaccine for oral delivery of Corona S1 and N-immune potent antigens. In addition, the team developed a commercial GMP standardized production method, including fermentation and protein collection. Proof-of-concept was achieved using an Avian coronavirus sequence for vaccination of chickens against the Avian Infectious Bronchitis (IBV) virus.
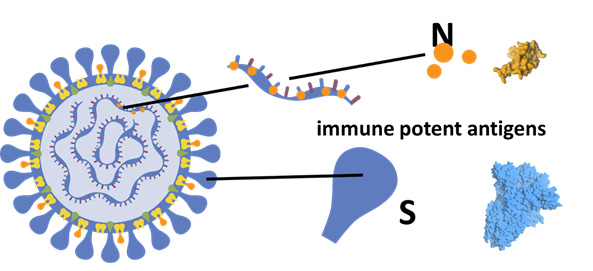
The main achievements of the program included:
- Definition of specific structural motifs of the corona S and N proteins, sufficient for induction of effective immune response.
- Development of three chimeric proteins for carrying three distinct structural domains, with enhanced mucosal activity for mucosal vaccination.
- Development of an E. coli-based protein expression system which produces and secretes the chimeric proteins to the growth media.
- Proof-of-concept for safety, specific antibody induction, activation of cellular immune response, and protection against challenge, was performed in two medium-scale animal studies, in chicken models and Avian corona H120 IBV.
- Development of fermentation media and process for protein production, following pharma regulations and GMP standards.
MIGAL is currently in the process of adjusting the genetic system to enable production of specific polypeptides of the recently-published Corona sequence. It has characterized the structural domains and is in the process of developing relevant chimeric proteins. Using its already-developed fermentation process, Migal believes that its proposed oral (mucosal) vaccination technology can be ready for testing recently.
MIGAL’s proposed COVID 19 vaccine is based on a new protein expression vector, which forms and secretes a chimeric soluble protein that delivers the viral antigen into mucosal tissues by self-activated endocytosis. In an In-Vivo study, it was demonstrated that the oral vaccination induces high levels of specific anti-IBV antibodies. Moreover, vaccinated birds were protected against challenge with a live virulent IBV strain, as demonstrated by rapid clearance of the virus.
Advantages of COVID-19 Subunit vaccine
- Cannot cause disease state
- Safe to use in immune suppressed patients
- Less chances of side-effects
- Oral administration
- Stimulation of cytotoxic T cells
- Relatively fast adaptation to new variant
Migal licensed the technology to MigVax Ltd fully owned subsidiary
Migal gave Exclusive license worldwide to MigVax Ltd fully owned subsidiary, to make, use and practice the Licensed Technology for the development, manufacture and commercialization Vaccine for viruses in Humans
Multidisciplinary Team
Research
Prof. Jacob Pitcovski Virology and poultry vaccination
Ehud Shahar, Ph.D. Immunology
Chen Katz, Ph.D. Microbiology
Itai Bloch Computational chemistry
Itamar Yadid, Ph.D. Microbial metabolic pathways
Management
David Zigdon Interim CEO
Prof. Itamar Shalit SAB Clinical
Ori Ben-Herzel VP BizDev
Rivka Zaibel, Ph.D. QA/RA consultant
Roni Pinkus, Ph.D. Production consultant
Sigal Tal, MD. VP Clinical
Ronald Ellis, Ph.D. CTO
The MIGAL Galilee Research Institute
The MIGAL Galilee Research Institute Ltd is a regional R&D center of the Israeli Science and Technology Ministry owned by the Galilee Development Company ltd. An internationally-recognized multi-disciplinary applied research institute, Migal specializes in biotechnology and computer sciences, plant science, precision agriculture and environmental sciences, and food, nutrition and health. Recognized as a powerhouse of applied research, for forty years MIGAL has cooperated closely with industry leaders, innovative startups, and technological accelerators. MIGALs' employees include 90 PhDs and 190 researchers distributed across 44 research groups, operating as an innovative research ecosystem that encourages collaboration across scientific, industrial, agricultural, academic and technological specialties.
From the press:
https://nocamels.com/2020/03/israeli-scientists-avian-vaccine-adapt-coronavirus
Videos
https://youtu.be/zjPKCSa24a8
https://www.ynet.co.il/articles/0,7340,L-5685810,00.html
https://13news.co.il/item/news/domestic/health/coronavirus-vaccine-1026258
